



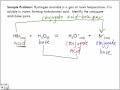














COMEDK 2012: The conjugate acid of NH2- is (A) N2H4 (B) NH4+ (C) NH2OH (D) NH3 . Check Answer and Solution for above question from Chemistry Conjugate Acid-Base Pairs. Acids and bases exist as conjugate acid-base pairs.The term conjugate comes from the Latin stems meaning "joined together" and refers to things that are joined, particularly in pairs, such as Brnsted acids and bases.. Every time a Brnsted acid acts as an H +-ion donor, it forms a conjugate base.Imagine a generic acid, HA. When this acid donates an H + ion to water Acid strength is determined by the amount of that acid that actually ionizes. Acids are molecular covalent compounds which you don't expect to ionize (release an #H^+# and leave behind the conjugate base, or #Cl^-# for example).. The strongest acids ionize 100%. There are 6 that most consider to be the "STRONG" acids: HCl, HI, HBr, HNO_3 #, H_2SO_4# and HClO_4#. In other words, a conjugate acid is the acid member, HX, of a pair of compounds that differ from each other by gain or loss of a proton. A conjugate acid can release or donate a proton. A conjugate base is the name given to the species that remains after the acid has donated its proton. The conjugate base can accept a proton. Conjugate acids and bases are part of the Bronsted-Lowry theory of acids and bases. According to this theory, the species that donates a hydrogen cation or proton in a reaction is a conjugate acid, while the remaining portion or the one that accepts a proton or hydrogen is the conjugate base. The conjugate base may be recognized as an anion. dict.cc | Übersetzungen für 'conjugate acid' im Isländisch-Deutsch-Wörterbuch, mit echten Sprachaufnahmen, Illustrationen, Beugungsformen, Conjugate Acid-Base Pair: Consider the weak monoprotic acid {eq}HA{/eq}. When this acid donates its proton, it becomes a weak base, {eq}A^-{/eq}. {eq}HA{/eq} and {eq}A^-{/eq}, are called conjugate Examples of how to use “conjugate acid” in a sentence from the Cambridge Dictionary Labs A conjugate acid is the particle produced when a base accepts a proton. The hydrogen sulfate ion is the conjugate acid of the sulfate ion. A conjugate base is the particle produced when an acid donates a proton. When acting as a base and reacting with water, Ammonia NH 3 can form a conjugate acid when it forms ammonium NH 4 +. In the reaction illustrated below, water serves both as acid and Anwendungsbeispiele für “conjugate acid” in einem Satz aus den Cambridge Dictionary Labs
[index] [2878] [6982] [8731] [125] [3010] [9431] [9724] [8011] [243] [2344]
Introduction to conjugate acids and bases. Created by Sal Khan.Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is... In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺).... Conjugate acid-base pairing is a concept which derives from the Broasted-Lowry Theory. In this section of Module 6: Acids and Bases in the HSC Chemistry Syll... All this talk of conjugate sounds scary! Not really, this video looks at what a conjugate base and acid are with multicoloured equations. Take a look to find... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Donate here: http://www.aklectures.com/donate.php Website video: http://www.aklectures.com/lecture/conjugate-acid-base-pairs Facebook link: https://www.faceb... Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together... Watch more videos on http://www.brightstorm.com/science/chemistrySUBSCRIBE FOR All OUR VIDEOS!https://www.youtube.com/subscription_center?add_user=brightstor... This chemistry video tutorial explains the concept of acids and bases using the arrhenius definition, bronsted - lowry and lewis acid base definition. It al...
Copyright © 2024 casinos.livesportshd.site